Biolog for
Sterility Assurance
Biolog Lab Services helps CSPs meet USP <797> Standards
USP-NF UPDATES
USP <797> Updates –
What this means for you.
USP <797> has been the guiding standard for pharmacies, hospitals, and healthcare facilities across the United States. Its mission? Ensuring that compounded sterile preparations (CSPs) are prepared and maintained to prevent harm to patients due to microbial contamination.
What’s new in the November 2023 USP <797> revision?
- Enhanced Sterility Assurance: The revised USP <797> raises the bar on sterility assurance, demanding stricter measures to prevent contamination and ensure safety.
- Harnessing Cutting-Edge Technology: It’s not just about “what” but “how.” The new USP <797> embraces modern technology, promising more precise, efficient, and reliable methods for microbial control.
- A Risk-Based Approach: One size doesn’t fit all. The revision introduces a risk-based approach, allowing tailored testing protocols based on different preparations’ unique characteristics and risks.
- Quality Control Evolution: Quality control becomes more robust and integrated, promising uncompromising standards throughout the compounding process.
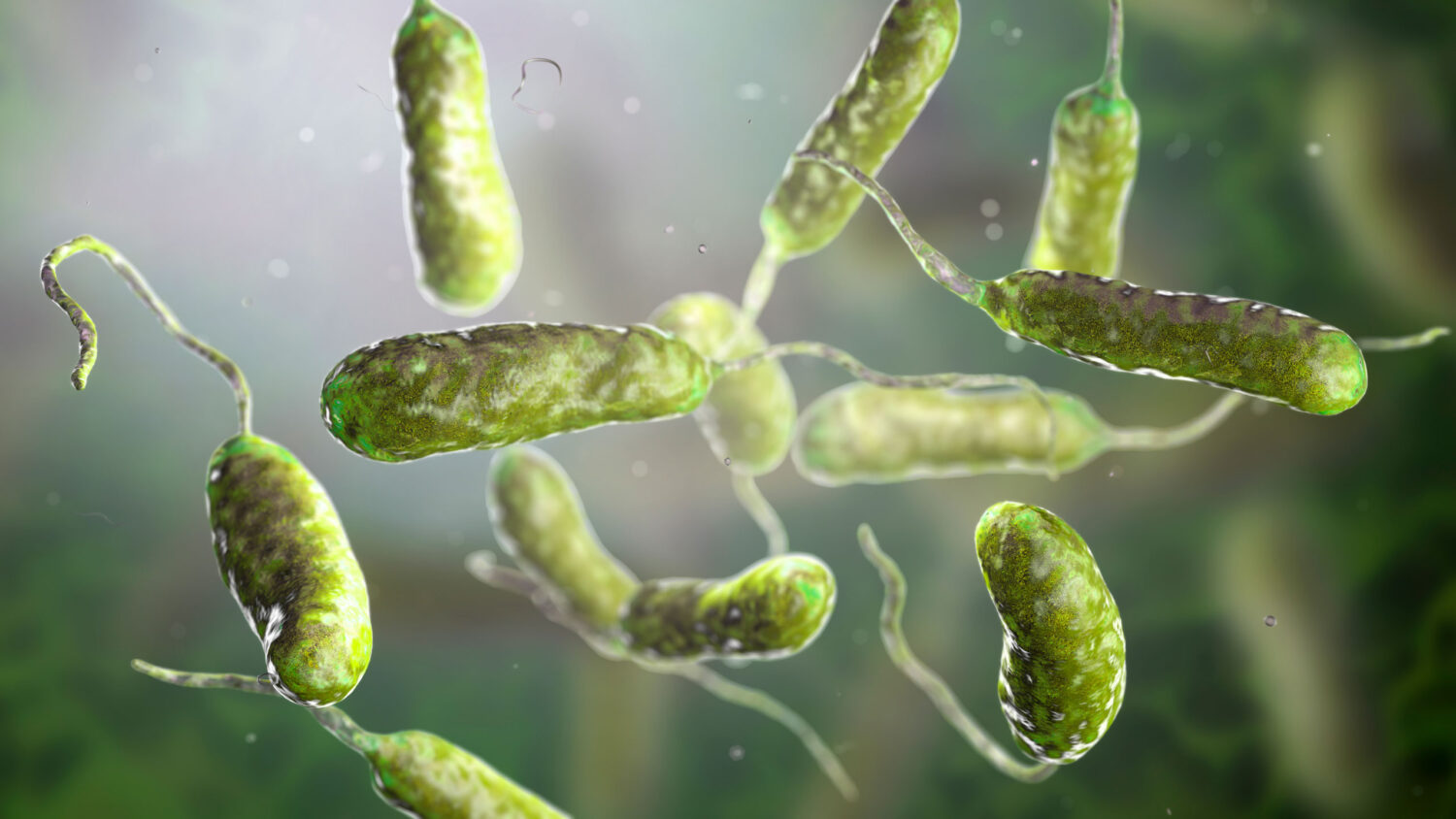
BACTERIAL IDENTIFICATION
USP <797> Bacterial Enumeration and Identification
Biolog Lab Services meticulously follows USP <797> guidelines, incubating samples at 30 – 35 ºC for 48 hours and 20-25 ºC for 5 days to detect a broad spectrum of microorganisms. Our advanced techniques ensure that every colony-forming unit is accurately enumerated and identified, leaving no room for ambiguity.
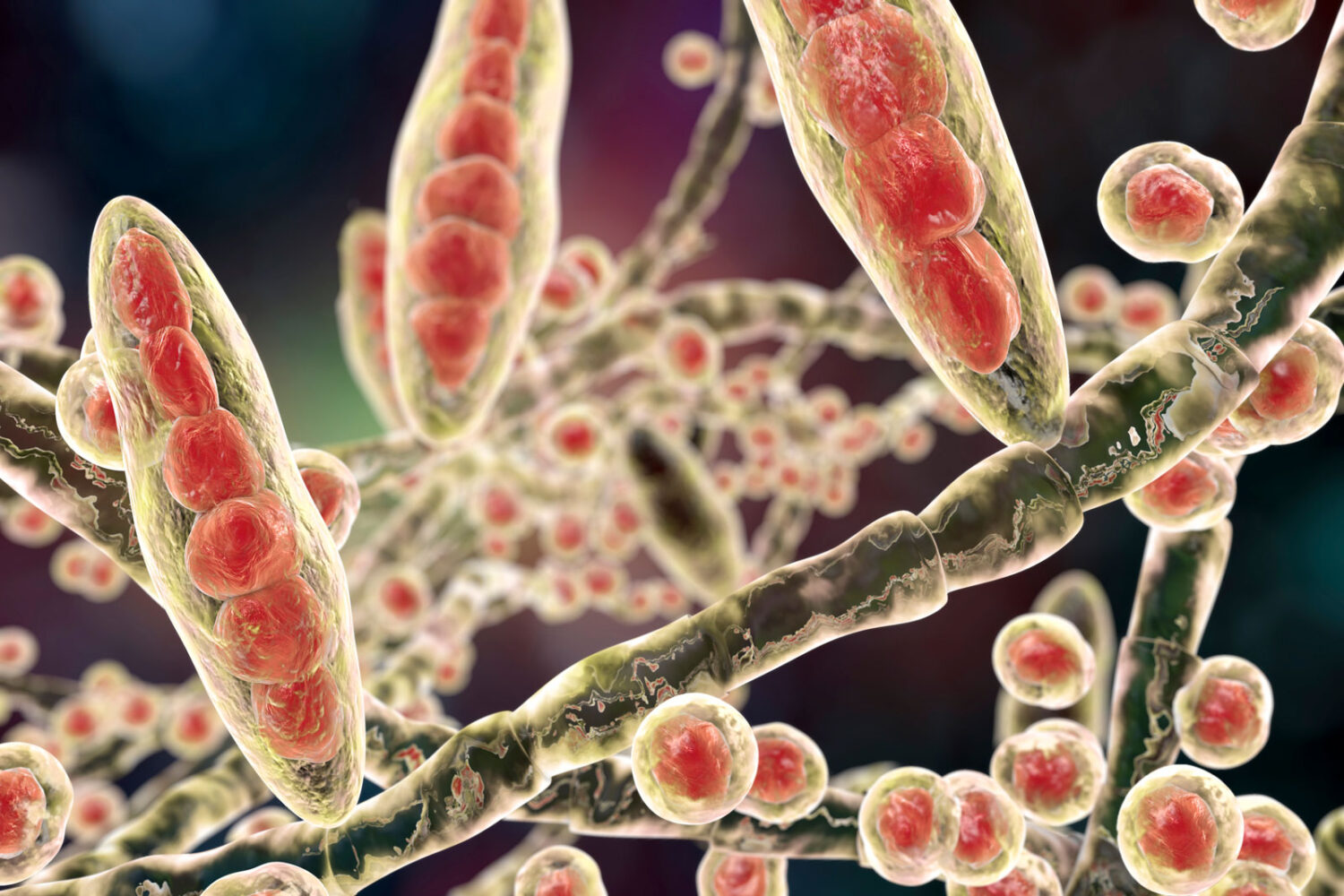
FUNGAL IDENTIFICATION
USP <797> Fungal Enumeration and Identification
We set the gold standard for fungal enumeration and identification. Under the direction of USP <797>, our dedicated team nurtures samples at 30 – 35 ºC for 48 hours and 20-25 ºC for 5 days to detect a broad spectrum of microorganisms. This gives you a comprehensive report detailing every fungal colony-forming unit’s enumeration and identification.
Gloved Fingertip Bacterial Sampling
Precision matters, especially when it comes to gloved fingertip sampling. Guided by USP <797> principles, we incubate at 30 – 35 ºC for 48 hours and 20-25 ºC for 5 days to detect a broad spectrum of microorganisms. This results in a thorough analysis that provides a precise enumeration of bacterial colony-forming units, offering you actionable insights.
Media Fill Test
The ultimate test of your facility’s integrity is here. Our lab experts incubate media-filled containers for at 20-25 ºC and 30 – 35 ºC for at least 7 days to detect a broad spectrum of microorganisms. Once incubation is complete, our detailed report highlights positive/negative results, providing vital information about visible turbidity.


